Ethics Committee
The Institutional Committee of Ethics in Research is an organization composed of multidisciplinary and independent professional members, who protect the rights, respect for the dignity, well-being and safety of patients who enter a research study.
Ethics Committee
The Institutional Committee of Ethics in Research is an organization composed of multidisciplinary and independent professional members, who protect the rights, respect for the dignity, well-being and safety of patients who enter a research study. This task involves the rigorous analysis of clinical trials, the assessment of the balance between risks and benefits, as well as the ethical and methodological evaluation of a research protocol.

The Institutional Ethics Committee evaluates the projects in the ethical principles:
- Respect for the autonomy of human beings
- Charity
- Justice
- No maleficence
The Ethics Committee conducts its evaluations under the principles of the Declaration of Helsinki, Guide of Good Clinical Practices and the Regulation of Clinical Trials in Peru.
Records of the Institutional Ethics Committee:
National Registration:
The Committee has registration code of INS RCEI-6.
International Registration:
The Ethics Committee is registered in the OHRP (Office of Human Research Protections) with institutional code IORG0001304 and code IRB00001733 (Institutional Review Board) both due 13 / sep / 2025. It also has the federal guarantee code FWA (Federalwide Assurance) ) FWA00001219 (due 31/ jan / 2029).
Network of Research Ethics Committees
Our Results:
We have the experience of having an autonomous and multidisciplinary team that guarantees good performance in the evaluation of clinical trials and observational studies as epidemiological, following bioethical principles and making a balance between the risks and benefits that the research subject will have.
In recent years we have evaluated clinical trials of recognized pharmaceutical laboratories such as Glaxo SmithKline, Novartis of Peru, Sanofi-Aventis, Pfizer, Merck Sharp & Dohme, Quintiles, AstraZeneca, Roche Products, Bristol Myers Squibb, Biogen Idec, Johnson & Jonhson, Cerexa Pharmaceutical, Takeda Pharmaceutical, Grunenthal, Quintiles, Bayer, ICON, PPD Peru SAC, Parexel, Inventiv Health, among others.
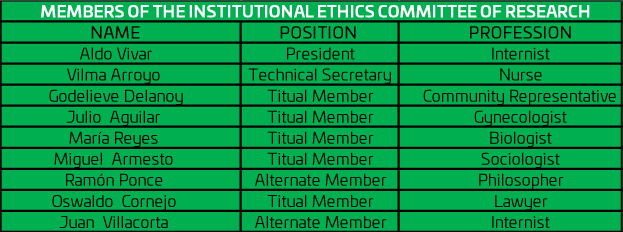
Directory of the Committee of Ethics in Research
The Board of Directors is made up of a multidisciplinary team.
DIRECTORY
For the execution of its functions, the Institutional Ethics Committee of Research is composed of eight members, only one of them is a substitute member, with no surveillance time limit.
Committee Functions
Administrative requirements for submitting an observational study
Documents to submit for review of an initial study:
Research protocol:
- Protocol (duly translated into Spanish)
Informed Consent and Patient Materials:
- Informed Consent (duly translated into Spanish)
- Patient Materials: surveys, questionnaires, informational material, etc. (Spanish version)
Documents of the principal investigator (PI) and research center:
- CV and simple copy of the degree or medical college ID and current medical team license.
- Training in Research Ethics and Training in Responsible Conduct for the team.
- Declaration of Primary and Secondary Investigator (Annex 5).
- Declaration of no conflict of interest from the PI and Secondary.
- Confidentiality declaration from the PI and Secondary (Annex 10).
Documents to submit for review of amendments:
- Amendments to the Protocol with change control. (Include a list of new changes)
- Clean version of Protocol Amendments.
- Amendments to the FCI with change control (include list with new changes)
- Clean version of FCI Amendments.
IMPORTANT:
All documents for review request and/or initial approval to the CIEI must be submitted with a letter addressed to Dr. Aldo Vivar Mendoza, Chair of the Ethics Committee, signed by the Principal Investigator conducting the study, and request the review. The submission letter must include the following information:
- Title of the EC.
- Name of the research product.
- Brief description and justification of the study.
- Duration of the study.
- Number of patients to enroll in Peru.
- Details of the research center.
* Deadline for document reception up to 8 days before the session..
Administrative requirements for submitting clinical trials to the CIEI
Documents to submit for review of an initial study:
Research protocol:
- Protocol English Version.
- Protocol Spanish Version.
- Affidavit of the Principal and Secondary Investigator (Annex 5).
- Confidentiality Agreement of Principal and Secondary Investigator (Annex 9).
- Declaration of No Conflict of Interest of Principal and Secondary Investigator (Annex 10).
Investigator’s Handbook:
- Investigator’s Manual English version.
- Researcher’s Manual Spanish version.
Informed Consent and Patient Materials:
- Informed Consent English Version.
- Informed Consent Spanish Version.
- Patient Materials/Surveys/Questionnaires. (Spanish Version)
Documents of the principal investigator and research center:
- CV including a simple copy of the degree or medical college ID and current license of the medical research team.
- GCP Certificate, Research Ethics Certificate, and Responsible Conduct Certificate for the entire team.
- Declaration of the Principal Investigator. (Annex 5).
- Confidentiality Declaration of the PI (Annex 9).
- Declaration of No Conflict of Interest of the PI (Annex 10).
- Valid insurance policy until the end of the study.
- Research Center’s Institutional Review Board (IRB) number.
- Operating authorization of the research center granted by the highest authority of the institution to which it belongs.
Documents to Submit for Amendments Review:
- Protocol Amendments with change control (include list of changes).
- Clean version of Protocol Amendments.
- FCI Amendments with change control (include list of changes).
- Clean version of FCI Amendments.
IMPORTANT:
Documents for review request and/or initial approval must be submitted with a letter addressed to Dr. Aldo Vivar Mendoza, Chair of the Ethics Committee, signed by the Principal Investigator conducting the study. The submission letter must include the following information:
- Title of the EC.
- Name of the research product.
- Brief Description and Justification of the study.
- Duration of the study.
- Number of patients to enroll in Peru.
- Details of the research center and IRB number.
* Deadline for receipt of documents up to 8 days before the session.
Schedule
The regular sessions of the Institutional Committee on Research Ethics (CIEI) are held on the Tuesday of each month; in which the research studies are reviewed. The deadline for delivery of the documents is five (05) days before each scheduled session in which the CIEI meets.
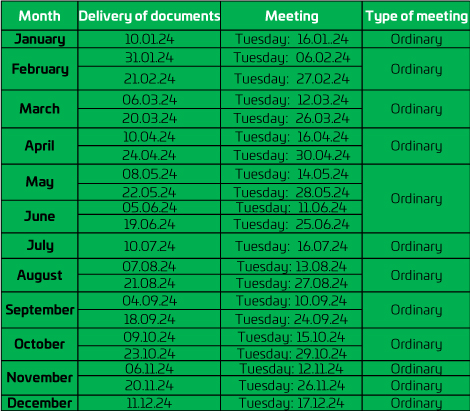
*Subject to variation prior confirmation the first days of each month.
PRICE LIST FOR REVIEW OF RESEARCH STUDIES
Initial Study Review – Clinical Study S/.2,400 consists of:
Principal Investigator Review S/. 1,200
It includes the review of the Informed Consent, Brochure, Curriculum of the Principal Investigator, Secondary Investigator and Coordinators and the continuous review during a year including visits if necessary.
Protocol Review S/. 1,200**
(This amount varies according to the number of centers that enter)
Includes initial review of the investigator protocol and
Determination of risk / benefit
Initial Study Review – Observational Study S/. 1,750
Annual renewal and Extension
- Annual study renewal S/. 1,200
- Time Extension Review S/. 800
Review of Amendments and additional documents
- Review of the Amendment to the Protocol S/. 750
- Review of the Amendment to Informed Consent S/. 750
- Review of materials delivered after the initial review (Such as: Brochure, questionnaires, guides, patient journals sent after the initial review) S/. 350
Changes in the Study:
- Administrative changes in the Informed Consent S/. 300
- Change of Investigator or Inclusion of New Secondary Researcher S/. 450
- Change of research center S/. 450
** The Cost of the Protocol is divided among the Study Centers.
All prices include IGV
CONTACT
From Monday to Friday:
- Morning: 9:00 a.m. to 1:00pm.
- Afternoon: 3:00 p.m. to 5:00 pm.
DELIVERY OF BILLS
- From Tuesday to Thrusday: 9:00 a.m. to 1:00 pm.
Institucional Ethics Committee of Research of Prisma NGO
Santo Toribio 115
Office 701, Lima – Perú
Mónica Mateo
E-mail: mmateo@prisma.org.pe
Tel. 2090400 Ext: 246
Cel. 955 346 426
Anita López
E-mail: alopez@prisma.org.pe
Tel. 2090400 Ext: 258
Cel. 955 368 591